The cooling rate of an object depends on many things. The size, composition, and initial temperature of the part and final properties are the deciding factors in selecting the quenching medium. A quenching medium must cool the metal at a rate rapid enough to produce the desired results.
Liquid Quenching:
The two methods used for liquid quenching are still-bath and flush quenching.
In Still-bath quenching, you cool the metal in a tank of liquid. The only movement of the liquid is that caused by the movement of the hot metal, as it is being quenched.
For flush quenching the liquid is sprayed onto the surface and into every cavity of the part at the same time to ensure uniform cooling. Flush quenching is used for parts having recesses or cavities that would not be properly quenched by ordinary methods. That assures a through and uniform quench and reduces the possibilities of distortion.
Quenching liquids must be maintained at uniform temperatures for satisfactory results. That is particularly true for oil. To keep the liquids at their proper temperature, they are usually circulated through water-cooled coils. Self contained coolers are integral parts of large quench tanks.
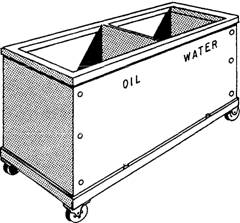
A typical portable quench tank is shown in Fig. This type can be moved as needed to various parts of the heat-treating shop. Some tanks may have one or more compartments. If one compartment contains oil and the other water, the partition must be liquid-tight to prevent mixing. Each compartment has a drain plug, a screen in the bottom to catch scale and other foreign matter, and a mesh basket to hold the parts. A portable electric pump can be attached to the rim of the tank to circulate the liquid. This mechanical agitation aids in uniform cooling.
i. Water:
It is probably the most widely used as it is simple and effective. Water can be used to quench some forms of steel, but does not produce good results with tool or other alloy steels. Water absorbs large quantities of atmospheric gases, and when a hot piece of metal is quenched, these gasses have a tendency to form bubbles on the surface of the metal. These bubbles tend to collect in holes or recesses and can cause soft spots that later lead to cracking or warping.
The water in the quench tank should be changed daily or more often if required. The quench tank should be large enough to hold the part being treated and should have adequate circulation and temperature control. The temperature of the water should not exceed 65°F.
When aluminium alloys and other non-ferrous metals require a liquid quench, you should quench them in clean water. The volume of water in the quench tank should be large enough to prevent a temperature rise of more than 20°F during a single quenching operation. For heavy-sectioned parts, the temperature rise may exceed , but should be kept as low as possible. For wrought products, the temperature of the water should be about 65°F and should never exceed 100°F before the piece enters the liquid.
Table :- Properties and Average Cooling Abilities of Quenching Media.
ii. Brine:
Brine is the result of dissolving common rock salt in water. This mixture reduces the absorption of atmospheric gases that, in turn, reduces the amount of bubbles. As a result, brine wets the metal surface and cools it more rapidly than water. In addition to rapid and uniform cooling, the brine removes a large percentage of any scale that may be present.
The brine solution should contain from 7% to 10% salt by weight or three-fourth pounds of salt for each gallon of water. The correct temperature range for a brine solution is 65°F to 100°F.
Low-alloy carbon steels can be quenched in brine solutions; However, the rapid cooling rate of brine can cause cracking or stress in high-carbon or low-alloy steels that are uneven in cross section.
Because of the corrosive action of salt on non-ferrous metals, these metals are not quenched in brine.
iii. Oil:
Oil is used to quench high-speed and oil-hardened steels and is preferred for all other steels provided that the required hardness can be obtained. Practically any type of quenching oil is obtainable, including the various animal oil, fish oils, vegetable oils, and mineral oils. Oil is classed as an intermediate quench. It has a slower cooling rate than brine or water and a faster rate than air. The quenching oil temperature should be kept within a range of 80°F to 150°F. The properties and average cooling powers of various quenching oils are given in above table.
Water usually collects in the bottom of oil tanks but is not harmful in small amounts. In large quantities it can interfere with the quenching operations; for example, the end of a long piece may extend into the water at the bottom of the tank and crack as a result of the more rapid cooling.
Non ferrous metals are not routinely quenched in oil unless specifications call for oil quenching.
iv. Caustic Soda:
A solution of water and caustic soda, containing 10 percent caustic soda by weight, has a higher cooling rate than water. Caustic soda is used only for those types of steel that require extremely rapid cooling is NEVER used as a quench for non-ferrous metals.
Warning:
Caustic soda requires special handling because of its harmful effects on skin and cloth.
v. Dry quenching:
This type of quenching uses materials other than liquids. Inmost cases, this method is used only to slow the rate of cooling to prevent warping or cracking.
vi. Air:
Air quenching is used for cooling some highly alloyed steels. When you use still air, each tool or part should be placed on a suitable rack so the air can reach all sections of the piece. Parts cooled with circulated air are placed in the same manner and arranged for uniform cooling. Compresses air is used to concentrate the cooling on specific areas of a part. The airline must be free of moisture to prevent of the metal.
Although non-ferrous metals are usually quenched in water, pieces that are too large to fit into the quench tank can be cooled with forced air drafts; however, an air quench should be used for non-ferrous metal only when the part will not be subjected to severe corrosion conditions and the required strength and other physical properties can be developed by a mild quench.
vii. Solids:
The solids used for cooling steel parts include cast iron chips, lime, sand, and ashes. Solids are generally used to slow the rate of cooling; for example a cast-iron part can be placed in a lime box after welding to prevent cracking and warping. All solids must be free of moisture to prevent uneven cooling.

No comments:
Post a Comment